In Angewandte Chemie International Edition, M. Kevin Brown and co-workers from Indiana University Bloomington reported a type of Suzuki coupling where 10% (Xantphos)CuCl catalyzed reactions between arylboronic esters and aryl iodides to provide biaryls. As in Suzuki coupling, a base was used, and in this case, the choice fell to sodium tert-butoxide. Optimally, the reaction proceeded in toluene at 80 ºC in 15 hours. Both electron-rich and electron-poor iodides and boronic esters were suitable for this process, but steric hindrance in one of the two starting materials needed Cy3PCuCl as the catalyst.
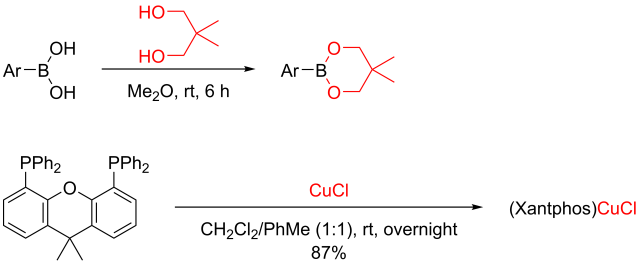
The arylboronic esters can be synthesized from the corresponding arylboronic acids and neopentyl glycol as reported by Aiwen Lei and colleagues from Wuhan University, and (Xantphos)CuCl can be made from CuCl and Xantphos as described by the laboratory of Yasushi Tsuji from Kyoto University. (See the scheme below.) Sodium tert-butoxide can be purchased from chemical companies.
Teleportation gates:
-
Article: Zhou, Y.; You, W.; Smith, K. B.; Brown, M. K. Angew. Chem., Int. Ed. 2014, 53, 3475–3479. DOI: 10.1002/anie.201310275.
- Indiana University Bloomington.
- M. Kevin Brown’s research group.
- Suzuki coupling on Wikipedia.
- Syntheses of arylboronic esters: Liu, Q.; Li, G.; He, J.; Liu, J.; Li, P.; Lei, A. Angew. Chem., Int. Ed. 2010, 49, 3371–3374. DOI: 10.1002/anie.201000460.
- Synthesis of (Xantphos)CuCl: Semba, K.; Fujihara, T.; Xu, T.; Terao, J.; Tsuji, Y. Adv. Synth. Catal. 2012, 354, 1542–1550. DOI: 10.1002/adsc.201200200.